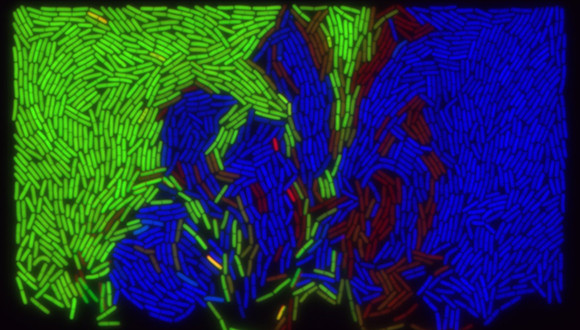
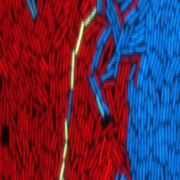
How do viruses choose whether to become nasty or not?
Researchers from the Shmunis School of Biomedicine and Cancer Research at TAU discovered a mechanism used by viruses to decide whether to kill their bacterial host. Interestingly, this mechanism involves a system that's intended to defend the bacteria from viruses.
Bacteriophages, also known as phages, are types of viruses that infect bacteria and use the microbes to replicate and spread. Even though the meaning of the word bacteriophage is "bacteria devouring" in ancient Greek, many phages can switch between two very different modes of action. One is the violent "bacteria eating" mode, in which the phage rapidly replicates inside the bacteria until the cell explodes and the virus moves to its next victim. The second is a "sleep mode", in which the virus incorporates itself into the bacterial genome and remains dormant. In fact, in this mode of action, a phage can even have a symbiotic relationship with its host bacterium.
Now, researchers from the Shmunis School of Biomedicine and Cancer Research at the Tel Aviv University were able to decipher a novel complex decision-making process that helps the virus to choose whether to turn nasty or not. In a study published in Nature Microbiology, they describe how the virus co-opts a bacterial immune system, intended to combat viruses like itself, in this decision-making process.
Using phage to understand viruses
"This finding is important for different reasons. One reason is that some bacteria, such as those causing the cholera disease in humans, become more violent if they carry dormant phages inside them - the main toxins that cause us harm are actually encoded by the phage genome," explains Prof. Avigdor Eldar, the senior researcher who led the study. "Another reason is that researchers are interested in phages and their modes of action as a novel way of attacking pathogenic bacteria. Finally, we are also hoping to use phage research to better understand viruses in general. Many human-infecting viruses can also alternate between dormant and violent modes".
In general, Eldar explains that phages usually prefer to stay in the safe, sleep mode, in which the bacteria "cares" for their needs and helps them safely replicate. Previous research published by the Eldar lab has shown that the phages use two kinds of information to decide whether to stay dormant or turn violent. The first type of information is the "health status" of the host. The virus monitors the damage accumulating on the bacterial DNA to determine if the bacteria is nearing death, prompting the virus to potentially seek a new host.
Prof. Avigdor Eldar from the Shmunis School for Biomedicine and Cancer Research, TAU
The second, and surprising finding, originally made by a group from the Weizmann Institute, was that phages can also communicate with other phages infecting other cells, by forcing their host to secrete a molecular signal and a receptor that can sense it: "a phage can't infect a cell already occupied by another phage. If the phage identifies DNA damage in its host but receives signals indicating the presence of phages in the area, it will choose to stay with its host and hope it will recover. If there is no signal, the phage ‘understands’ that there might be room for it in another host nearby and it will turn violent, replicate quickly, kill the host and move on to the next target," Eldar explains.
The new study deciphers the mechanism that enables the virus to make these decisions. "We discovered that in this process the phage actually uses a system that the bacteria developed to kill phages," says Polina Guler, a PhD student in Eldar's lab who led the study. The research of bacterial defense systems against phages has exploded in recent years. The most famous of such systems is the transformative gene-editing tool Crispr-cas9, but today there are more than 150 different defense systems known to science. "Bacteria and phages are engaged in an evolutionary arms race," says Guler
In this study, Guler, Eldar and their colleagues found that the phage developed two strategies to respond to a specific defense system and to use this system as part of its decision-making process. If it does not sense a signal from other phages - indicating it has a good chance of finding new hosts - it activates one mechanism that disables the defense system. "The phage switches to its violent mode, and with the defense system neutralized, it is able to replicate and kill its host," describes Guler. "If the phage senses high concentrations of the signal, instead of disabling the defense system, it evades it by turning on its dormant mode".
New level of sophistication
"The research revealed a new level of sophistication in this arms race between bacteria and viruses," adds Eldar. Most of the bacterial defense systems against phages were studied in the context of viruses that are always violent. Far less is known about the mechanisms of attacks and interaction with viruses that have a dormant mode. "The bacteria also have an interest in keeping the virus in the dormant mode, first and foremost to prevent their own death, and also because the genes of the dormant phage might even contribute to bacterial functions," says Eldar.
Now researchers in his lab are trying to decipher how these attack and defense systems are functioning in other types of bacteria and viruses and better understand this multilayered evolutionary arms race. "For example, we are testing if bacteria in nature can manipulate viruses to stay in the dormant mode by mimicking the signals the viruses use to communicate. In this struggle, every sensing mechanism improves the ability to respond to the environment, but can also serve as an Achilles' heel, which your opponent can use against you," summarizes Eldar.
Recently the Eldar lab was awarded the prestigious ERC synergy grant from the European union. This 8-million-euro grant was awarded in collaboration with two additional labs from England and Spain. "The grant aims to expand our understanding of how phages utilize communication to adapt to complex bacterial communities", he says.