Electric Body Parts: Charged Nanoparticles Can Lead Medications to Specific Organs
Scientists in Dan Peer’s lab found that positively charged mRNA nanoparticles concentrate in the lungs - suggesting new treatments for lung cancer
The mRNA revolution gained speed and worldwide fame thanks to the COVID-19 pandemic. However, antiviral vaccines are just the tip of the iceberg for using mRNA wrapped in microscopic fat bubbles (called lipid nanoparticles - LNPs) to change the function of specific cells and tissues in the body. Yet, realizing this potential requires making sure the LNPs reach the right places in the body.
From Vaccine Vehicles to Precision Medicine
In a new study, published in the scientific journal ACS Nano, researchers in Dan Peer’s lab at Tel Aviv University's Shmunis School of Biomedicine and Cancer Research discovered that tweaking the composition of an LNP can give it a slight positive electric charge, and that these charged particles concentrate in the lungs, unlike other LNPs that tend to reach mainly the liver and spleen. They also showed how such LNPs can be used to carry mRNA that induces toxin production, effectively eliminating lung tumor cells - indicating the discovery can be used to develop new, specific, and effective therapies for hard-to-treat lung cancer and lung metastases.
Dr. Gonna Somu Naidu and Dr. Riccardo Rampado, post-doctoral researchers who led the study in the Peer lab, explain that their research started from practical, mechanistic reasons. "LNPs are made of combinations of different short fat molecules called lipids that self-assemble as a protective bubble around the mRNA molecule as a result of their chemical qualities, such as their hydrophobic (water-averse) nature that pushes them to form shapes that minimize their exposure to water," explains Dr. Rampado.
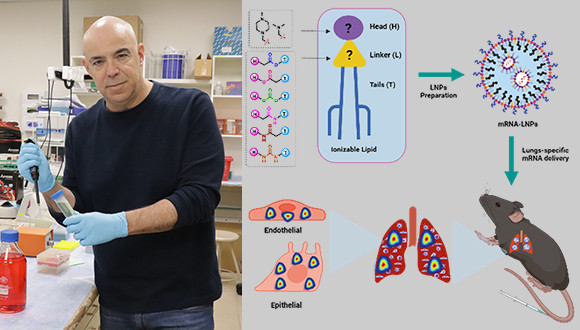
Selecting the right types and combination of lipids determines whether the mRNA will enter cells and be successfully translated into proteins. "The LNPs need to remain stable in production, in the vials until they are injected into the body, and as they flow through the bloodstream. When they enter the cells, it is usually through the endosome, the cell’s 'digestive organ'," says Dr. Rampado. Then, the mRNA must escape the endosome to the cell’s cytoplasm, where it can be translated into proteins. "To make sure this happens, we design the LNPs with ionizable lipids; as they move from the bloodstream to the endosome, which has a more acidic environment, they become positively charged. This positive charge causes them to interact with the endosomal membranes, disrupt them, and deliver the mRNA to the rest of the cell," adds Dr. Naidu.
Tweaking Chemistry to Target Tumors
The new study began with a large-scale effort to optimize the ionizable lipids needed both to enable this complex cellular process and to remain stable in mass-scale production for clinical use. "We created and screened a lipid library focusing on a less-studied aspect of the lipids - the linker molecules connecting the head and the tail of each lipid," says Dr. Naidu. To their surprise, they discovered that LNPs with ionizable lipids containing amide and urea linkers tended to concentrate in the lungs.
"To understand what drives these specific LNPs to reach the lungs, we conducted sophisticated statistical analyses of particle characteristics such as size and electric charge and realized that the slight positive charge determines the destination of the LNPs in the body," says Dr. Rampado. They then used animal models that enabled them to see which lung cells are transfected by the charged LNPs. "We found that all the different lung cells - epithelial, endothelial and immune cells - took up the nanoparticles and expressed the mRNA they carried. It shows that effective cell transfection is not restricted to receptors on a specific cell type." Finally, by using charged LNPs carrying mRNA encoding a bacterial toxin, they successfully treated mice with melanoma lung metastases, reduced tumor size, and extended survival.
"Our discovery shows how, by making tiny chemical changes, we can alter the properties and behavior of LNPs, opening the way for the development of new therapies for lung tumors. More broadly, our research demonstrates how changes in electric charge can lead LNPs to concentrate in specific tissues. In combination with other techniques for producing cell-type-specific LNPs, this approach opens the door to a new generation of precision mRNA medicine," concludes Prof. Peer, summing up the far-reaching implications of the discovery.